
Engineering the Future
of Medicine for
Women, Today
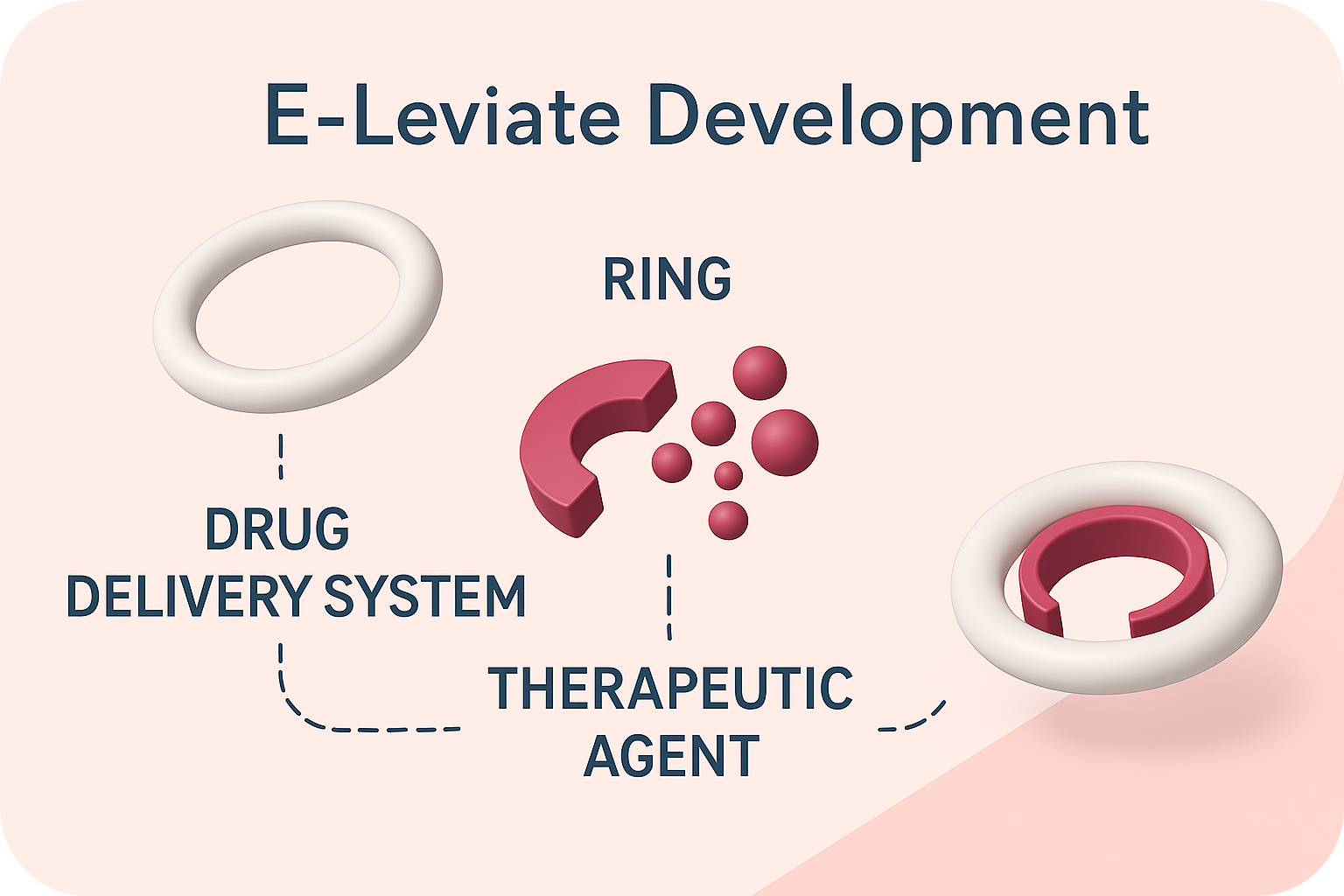
E-Leviate is a newly developed, next-generation medical device designed to treat vaginal atrophy, genitourinary syndrome of menopause (GSM), pelvic organ prolapse (POP), urinary stress incontinence, and cystitis. E-Leviate Pharma Ltd leverages proprietary, IP-protected technology to deliver cutting-edge solutions in drug delivery and biomedical innovation.



